air classifying grinder

air classifying grinder
——Flue gas deacidification process sodium bicarbonate grinding machine
Hazardous waste treatment Steel plant coke oven Thermal power generation municipal waste Sludge incineration non-metallic minerals Ceramic industry glass manufacturing
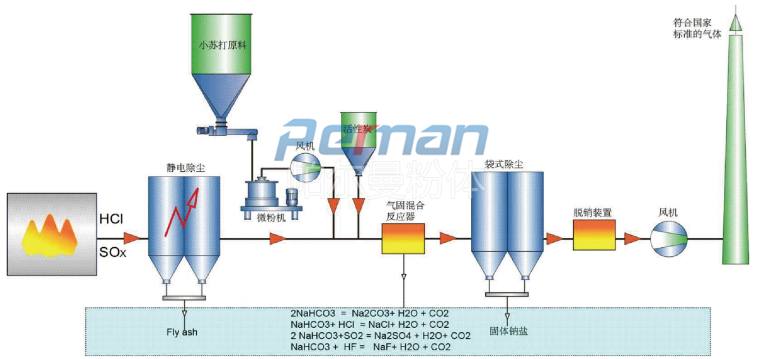
With the increasingly stringent requirements of environmental protection regulations, the process based on the baking soda dry deacidification principle developed by Perlman in conjunction with advanced European and American processes has been increasingly used. Various pollutants contained in flue gas are removed by dry adsorption using sodium bicarbonate as adsorbent. Its purification effect is comparable to other known methods, such as the spray adsorption method using lime milk as the adsorbent. Dry flue gas purification can not only be used in coal power plants, hazardous waste treatment, municipal waste or alternative fuel incineration plants, but can also be widely used in industrial furnaces in glass, cement, metallurgy and other industries. Dry flue gas purification can economically remove gases containing acidic substances, such as SO2, HCI, etc., and meet the national flue gas emission standards.
Flue gas contains a large amount of acidic gases. After a large amount of data and experiments, it has been found that only when sodium bicarbonate (baking soda, NaHCO3) is small enough to a certain extent, can it react with the acidic components in the flue gas with maximum efficiency. It removes acidic pollutants in flue gas through chemical adsorption, and can also remove some inorganic and organic trace substances through physical adsorption. This process sprays sodium bicarbonate fine powder directly into high-temperature flue gas. At high temperatures, sodium bicarbonate decomposes to produce sodium carbonate Na2CO3, H2O and CO2.
Typically, with flue gas temperatures between 140 and 250 °C, a slight excess of sodium bicarbonate (stoichiometric factor between 1.1 and 1.3) is usually sufficient due to the high activity of the sodium bicarbonate adsorbent.

